One of the most significant milestones in drug development is moving to first in human clinical trials. It’s the first opportunity for a sponsor to evaluate the new chemical entity or biologic’s impact in humans. You might need to assess the safety and tolerance of the drug, determine pharmacokinetics, identify early pharmacological activity relative to exposure level, assess any observed effects on subsets of study participants, and evaluate therapeutic outcomes in a smaller group of patients that suffer from the targeted disease. Here are six steps to help your organization reach its goals.
Which Regulatory Interactions Are Necessary?
Preparing for regulatory interactions during early clinical development is crucial. You will need to clarify any uncertainties that regulatory agencies might have when looking at your FIH program. The trial design should show a strategy for proving safety and proactively addressing any risks. This includes clinical conduct and reporting. Use a well-documented scientific rationale to support the plan alongside the Investigator's Brochure (IB). All of this is necessary for an Investigational New Drug application. Scheduling a pre-IND meeting with the FDA can be beneficial, and the feedback can affect your trial design.
Determining a Starting Dose
The starting dose can be where a FIH study fails or succeeds. It needs to mitigate the risk of toxicity while still having observable pharmacologic activity. The wrong dose or escalating too slowly can cause an increased study size, higher costs, and longer timelines. Escalating too quickly or too high of a dose can lead to health and safety concerns.
Designing the Trial
The two most common trial designs are single ascending dose and multiple ascending dose. FIH trials are usually dose-escalation studies, receiving increasing doses of the investigational product in different stages. The dose can increase once the Safety Review Committee confirms safety, PK, or other protocol-specific requirements.
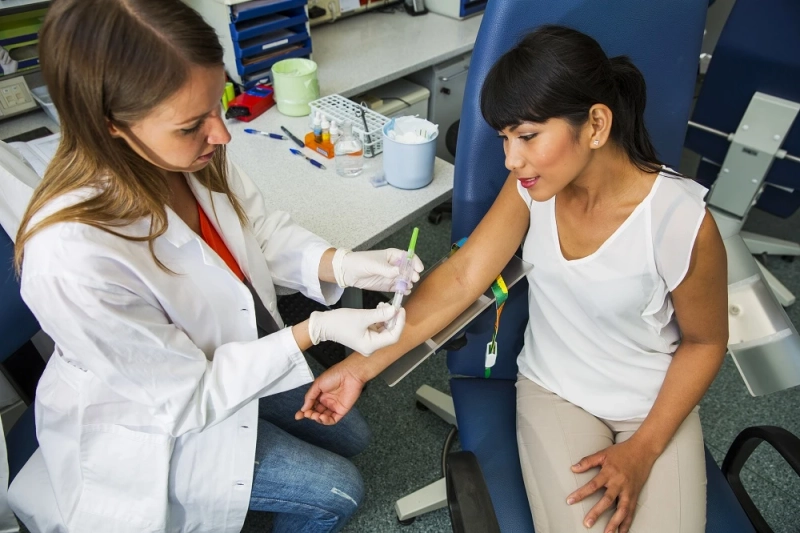
Participant Safety
You must give detailed consideration to risks in early phase trials. IRBs assess whether the risk to participants has been minimized and if the risks are justifiable. Potential participants must be aware of the risks and provide informed consent to participate in the trial. IRBs will also review safety monitoring plans, so be sure to make them a priority.
Recruiting Potential Participants
Potential participants should be as well informed as possible. This can be an interesting challenge when recruiting for first-in-human clinical trials, given the lack of data on your drug. It is beneficial to partner with a contract research organization with experience and large study participant databases, and can guide you throughout the process.
Managing Resources and Conducting the Study
Finally, you will need to plan resources and conduct the study. You might need specific equipment and customized meals to conduct an effect test. You might need cognitive test batteries for CNS-active drugs. Plus, you also need the proper resources to produce the drug. Finding a CRO with CDMO pharma capabilities can help you get access to the resources you need and have a partner that can assist with conducting the study. The CRO/CDMO likely has access to the resources you need for your FIH study, from trained staff to proper equipment.
About Altasciences
Are you looking for a CRO/CDMO drug development partner? Altasciences, a mid-sized contract research organization, is the partner with the expertise you need to make your next project a success. As an integrated CRO with pharmaceutical CDMO services, Altasciences offers partners more than 25 years of research experience for preclinical studies and clinical trials. This CRO uses an innovative, integrated approach that pharmaceutical and biotechnology companies can rely on. Altasciences also offers partners expertise in a wide range of study types and therapeutic indications. This includes a wealth of experience in first-in-human clinical trials and CNS clinical trials. Partners also gain access to essential resources, including experienced and highly trained staff, a recruiting database of over 400,000 potential participants, and over 580 beds for overnight studies. Choose this trusted CRO/CDMO for all your early clinical development needs.
Partner with Altasciences for help with your first-in-human clinical study at https://www.altasciences.com/
Original Source: https://bit.ly/3Fqr5G6
6 Steps to Planning Your First-In-Human Clinical Trial
First in human clinical trials are a crucial part of drug development.