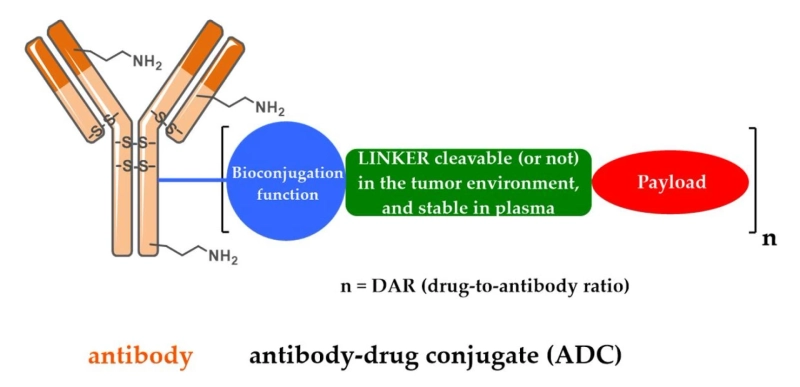
The antibody-drug conjugates (ADCs) market is a fast-growing segment within the pharmaceutical industry. A targeted therapy that combines the specificity of monoclonal antibodies with the cytotoxic effects of chemotherapy drugs, ADCs are designed to selectively deliver potent anticancer drugs to tumor cells and cause minimal damage to healthy tissues. Their importance lies in their ability to enhance treatment efficacy, reduce systemic toxicity, overcome drug resistance, and provide personalized treatment options for patients.
The antibody drug conjugates market has witnessed significant growth in recent years and is expected to continue expanding. There is an increased adoption and availability of ADC therapies in various regions. North America has historically been a dominant market for ADCs, but there is growing market penetration in Europe, Asia Pacific, and other regions. According to Allied Market Research, the global antibody drug conjugates market is estimated to reach $3,198 million by 2023, registering a CAGR of 12.9% during the forecast period 2017 to 2023. Several factors contribute to this growth, including the increasing incidence of cancer worldwide, the demand for more cost-effective and targeted cancer therapies, and advancements in biotechnology and antibody engineering.
The ADCs market is highly competitive, with numerous pharmaceutical and biotechnology companies actively engaged in research and development activities. These companies are focusing on expanding the application of antibody drug conjugates to different types of cancer and exploring combination therapies with other treatment modalities. The antibody drug conjugates market witnessed several significant developments in recent years. Here are a few of them:
Antibody drug conjugates have been approved for new indications, broadening their usage beyond initial approvals. For example, Brentuximab vedotin (Adcetris) initially received approval for Hodgkin lymphoma and systemic anaplastic large cell lymphoma. However, it has since gained approvals for other indications, including cutaneous T-cell lymphoma and peripheral T-cell lymphoma. This expansion of indications showcases the versatility of antibody drug conjugates in addressing different cancer types. Antibody drug conjugates are being explored in combination with other treatment modalities to enhance their therapeutic potential. Combination therapies can improve treatment outcomes by targeting cancer cells through multiple mechanisms of action. For instance, the combination of ADCs with immune checkpoint inhibitors has shown promising results in preclinical and clinical studies, potentially improving response rates and patient outcomes. The development of new cytotoxic payloads and linkers has contributed to the improvement of antibody drug conjugates efficacy and safety. Payloads with enhanced potency and mechanisms of action, such as DNA-damaging agents and microtubule inhibitors, are being incorporated into ADCs. Additionally, advances in linker technologies have enabled more stable attachment of payloads, ensuring controlled drug release and reducing off-target effects. Researchers are continuously identifying and validating new target antigens for antibody drug conjugates development. This expanding repertoire of target antigens allows for the development of ADCs against a broader range of cancer types. It also enables the potential for personalized medicine by matching specific antibody drug conjugates to patients with tumors expressing the corresponding antigens. Advancements in Antibody Engineering: The field of antibody engineering has seen significant advancements, enabling the design and optimization of antibody drug conjugates. Techniques such as antibody humanization, affinity maturation, and antibody fragment engineering have improved the specificity, binding affinity, and pharmacokinetic properties of antibodies used in ADCs. These advancements contribute to the development of more effective and targeted ADC therapies. Several ADCs have gained regulatory approvals and entered the market, expanding the available treatment options. For example, in recent years, antibody drug conjugates such as Sacituzumab govitecan (Trodelvy), Enhertu (fam-trastuzumab deruxtecan-nxki), and Blenrep (belantamab mafodotin-blmf) received approvals for the treatment of various types of cancers. These approvals demonstrate the increasing acceptance and clinical relevance of antibody drug conjugates. The antibody drug conjugates pipeline is robust, with numerous ADC candidates in various stages of development. Pharmaceutical companies and biotechnology firms are investing in the research and development of antibody drug conjugates, expanding the potential for new therapies. The pipeline includes ADCs targeting different cancers, utilizing diverse payloads and conjugation technologies.All in all, the antibody drug conjugates market has seen significant developments that contribute to the advancement of ADC therapies, offering new treatment options and improving patient outcomes in the field of oncology.