Background and overview
2-Pyrrolidone is miscible with water, alcohol, ether, chloroform, benzene, ethyl acetate and carbon disulfide; it is insoluble in petroleum ether, and its main use is as polyvinylpyrrolidone (PVP) monomer N-vinylpyrrolidone (NVP) ) raw material, and it is also a high-grade solvent, which is used in the production of pharmaceuticals, resins, acetylene recovery, floor wax special inks, etc. NVP can be prepared by the reaction of 2-pyrrolidone and acetylene, and then polymerized to obtain PVP. PVP has excellent solubility, low toxicity, film formation, complexation, surface activity and chemical stability, and is widely used in medicine, food, daily In the fields of chemicals, coatings, polymer polymerization, etc., it also has many uses in textile, printing and dyeing, papermaking, photosensitive materials, agriculture and animal husbandry. There are three main industrial production routes for 2-pyrrolidone: 1) Rapoo method: the raw material acetylene and formaldehyde are first reacted to generate 1.4-butynediol, hydrogenated to 14-butanediol, converted into γ-butyrolactone, and then combined with 1.4-butynediol. Ammonia reaction produces 2-pyrrolidone. 2) Recently, due to the successful development of a new process of butane oxidation to synthesize or 1.4-butanediol, γ-butyrolactone has a rich source, so it has become the main route for the production of pyrrolidone series products. 1.4-succinonitrile should be obtained, which is reduced to aminobutyronitrile by partial hydrogenation, and then hydrolyzed and cyclized to 2-pyrrolidone. This route was successfully developed and industrialized by the Dutch company DSM in the late 1970s.
Application
2-pyrrolidone can be used as a high boiling point solvent for petroleum processing; prepare N-vinylpyrrolidone, acrylonitrile, aminobutyric acid; also be used as a plasticizer for coatings; under the action of benzoyl chloride and alkali metals, it can be polymerized to form nylon -4 etc. 2-Pyrrolidones are a class of pyrrolidine compounds with a five-membered lactam ring, which are widely found in natural products and various synthetic compounds. Since pyrrolidones have various biological activities and are widely used in the field of medicine, many compounds with various biological activities can be prepared by modifying and functionalizing the structure of pyrrolidones.
1. 2-Pyrrolidone ring substitution product
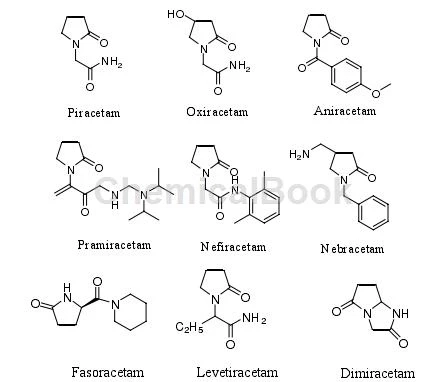
The 1-position of 2-pyrrolidone is a nitrogen atom. By substituting various groups on the nitrogen atom, a wide range of pharmacological activities can be obtained. The most classic example is the invention of the nootropic drug piracetam. Piracetam, a drug developed in the early 1970s to treat memory impairment and improve cognitive ability in patients with senile dementia, can be said to be the first pyrrolidone compound used in clinic. The characteristics of small side effects and non-addictive properties make people have great interest in the development of pyrrolidones with pharmacological activity. Piracetam is a derivative of γ-aminobutyric acid. Later, using the structure of piracetam as a model, a large number of drugs for improving brain function were developed by changing the substituents on the 1 and 4 positions on the 2-pyrrolidone ring. , the representative drug and its structure are shown in the figure:
2. Substitution products at the 1 and 3 positions on the 2-pyrrolidone ring
In most cases, in addition to the group substitution on the 1-position nitrogen atom of the 2-pyrrolidone ring, substitution at other positions in the ring can obtain a wider range of pharmacological activities. For example, in recent years, chemists and pharmacologists have shown increasing interest in compounds containing an α-methylene-γ-butyrolactam backbone. This is mainly because, like α-methylene-γ-butyrolactone, α-methylene-γ-butyrolactam has physiological activities such as cytotoxicity, anti-tumor and anti-inflammatory. Studies have shown that α-methylene-γ-butyrolactone is too toxic to be used clinically. Compared with α-methylene-γ-butyrolactam, the toxicity is much less, so it has The possibility of clinical application in cancer treatment. Compounds with α-methylene-γ-butyrolactam skeleton are continuously isolated from terrestrial and marine natural products. In addition to efforts to find compounds with this backbone in natural products, chemists are taking different routes to synthesize α-methylene-γ-butyrolactam derivatives with different structures for pharmacological screening.
3. Substituted products at 1, 4 positions on the 2-pyrrolidone ring and their synthesis
Substitution at the 4-position of 2-pyrrolidone can obtain many pharmacologically active compounds. A more classic example is the design of some anti-HIV drugs. Generally, the design of anti-HIV drugs can start from three aspects: (1) Prevent the virion and T-4 lymphoid Binding of cells; ⑵ inhibition of viral reverse transcriptase; ⑶ inhibition of HIV virus protease. Existing antiviral drugs can be divided into two categories: reverse transcriptase inhibitors and protease inhibitors, wherein reverse transcriptase inhibitors can be divided into nucleoside reverse transcriptase inhibitors and non-nucleoside reverse transcriptase inhibitors. Since the first anti-HIV drug zidovudine was launched in the 1980s, the initial clinical use was basically a single drug to treat patients with HIV disease, but its efficacy was not good, and then a combination of drugs was often used. ,Significant effect. In view of the increasing drug resistance of HIV virus, it is urgent to develop new anti-HIV drugs. A series of phenyl-substituted 2-pyrrolidone compounds have been designed and synthesized, and their anti-HIV activity has been investigated in order to discover a class of effective reverse transcriptase inhibitors.
4. Substitution products at the 1,5 position of 2-pyrrolidone
The 2-pyrrolidone derivatives that can be used as potassium channel blockers have been successfully synthesized, and their general structural formula is shown in the figure
Such compounds are particularly suitable as antiarrhythmic active ingredients, especially for the treatment and prevention of atrial arrhythmias, such as atrial fibrillation (AF) or atrial flutter.
5. Substitution products of multiple positions on the 2-pyrrolidone ring
In most cases, the substitution of multiple positions on the 2-pyrrolidone ring can meet the needs of a wider range of pharmacological activities. Monoamine oxidase (MAO, EC) is an oxidative deamination that causes endogenous monoamine neurotransmitters such as dopamine, serotonin, epinephrine or norepinephrine and trace amounts of amines such as phenethylamine as well as large amounts of exogenous amines The role of flavomycin. A patent was invented by some research, and a series of derivatives of pyrrolidone that can be used as MAOB inhibitors were synthesized, and the general structural formula is shown in the figure.
Preparation
2-pyrrolidone is produced by hydrocyanic acid and acrylonitrile. Hydrocyanic acid (HCN) reacts with acrylonitrile (AN) to prepare 2-pyrrolidone. The reaction process is:
1) The addition reaction of HCN and AN is catalyzed by triethylamine to generate succinonitrile. The reaction was carried out at normal pressure and about 70 ℃, and the yield of succinonitrile was as high as 97%.
2) Partial hydrogenation and reduction of succinonitrile to aminobutyronitrile, the reaction uses carrier nickel as hydrogenation catalyst, the total pressure is 14.0 MPa, the temperature is 80~100 ℃, and liquid ammonia is used as the solvent, and the conversion rate of succinonitrile is close to 100%, Aminobutyronitrile selectivity 85 %.
3) γ-aminobutyronitrile hydrolysis and cyclization reaction generates 2-pyrrolidone. This reaction is carried out in a plug flow system at a temperature of 210 ℃, and the nitrile conversion rate and product selectivity are both close to 100%.
https://www.arshinepharma.com/info/application-of-2-pyrrolidone-74334463.html