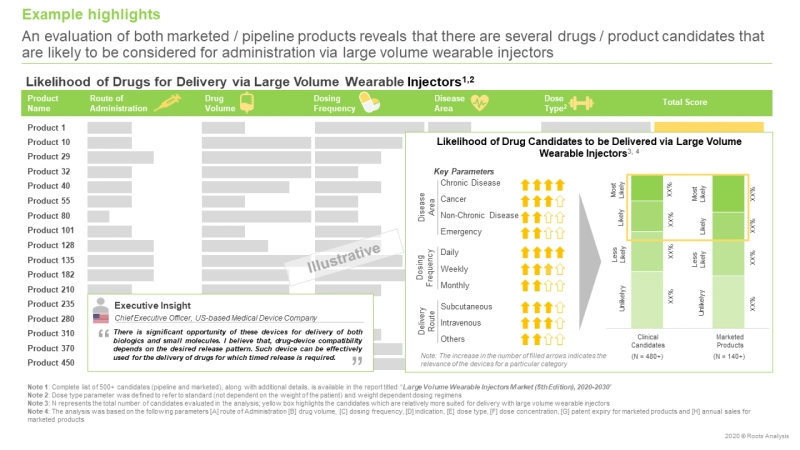
To meet the increasing demand for patient-friendly and safe options for drug administration, the drug developers are expected to broader their portfolio of drug candidates to make them favorable for delivery through self-injection systems. We identified several parameters which affect the likelihood of the drug to be delivered via the large volume wearable injectors. These include the drug’s volume and dosage, dose frequency, dose type, route of administration, target disease indication and year of patent expiry (in case of marketed drugs).
An analysis of over 800 marketed and pipeline drugs revealed that, presently, there are close to 200 molecules that have demonstrated the potential to be delivered via large volume wearable injectors. Of these, 90% are biologics and 10% are small molecules. In fact, in an interview with the chief executive officer of a US-based medical device company, he stated that “There is significant opportunity of these devices for the delivery of both biologics and small molecules. I believe that, drug device compatibility depends on the desired release pattern. Such device can be effectively used for the delivery of drugs for which timed release is required. ”
Based on our proprietary scoring criteria, subcutaneously delivered drugs have the highest likelihood to be administered via the large volume injectors. Self-subcutaneous route of administration is well accepted by the patients whereas IV infusion has to be done in a hospital setting. Moreover, majority of the large volume wearable injectors have been designed with the needle / cannula systems which can penetrate the epidermis to deliver the drug to the subcutaneous capillary and lymphatic vessels where it is readily absorbed. Further, a number of drugs are likely to lose marketing exclusivity due to patent expiry in the coming years. This paves the way for the entry of a large number of biosimilars which are reformulated to be administered via the subcutaneous route, presenting additional opportunities for the growth of the large volume wearable injectors market.
Further, the analysis revealed that drugs designed for specific clinical conditions, such as neurological disorders, oncological disorders, pain management, reproductive disorders and metabolic disorders, are most suitable for administration with large volume wearable injectors. Interestingly, several drug device combinations are already being developed / marketed for the aforementioned indications, at present. Notable examples include (in alphabetical order, no selection criteria) D-mine Pump (EVER Pharma / Sensile Medical), Lutrepulse System (Insulet / Ferring Pharmaceuticals) and Pushtronex System (Amgen / West Pharmaceutical Services).