Introduction:
Fourier Transform Infrared Spectroscopy (FTIR) is a powerful analytical technique used to identify organic, polymeric, and, in some cases, inorganic materials. It provides critical information about the molecular composition and structure of a sample, making it a valuable tool across diverse fields such as chemistry, materials science, forensics, and environmental science.
In this blog, we will explore the principles of FTIR, how it works, its applications, and the steps involved in interpreting FTIR spectra.
What is FTIR Spectroscopy?
FTIR is a type of infrared (IR) spectroscopy that uses the mathematical technique of Fourier transformation to convert raw data into an interpretable spectrum. The spectrum obtained provides a molecular "fingerprint" that helps identify and quantify compounds based on their characteristic absorption bands.
Key Features:
- Range: Mid-IR region (4000–400 cm<sup>-1</sup>)
- Output: Absorbance or transmittance spectra
- Principle: Absorption of infrared radiation at specific wavelengths by molecular bonds
How Does FTIR Work?
The Basics of Infrared Spectroscopy
Molecular Vibrations:
- When a molecule absorbs IR radiation, its bonds vibrate at specific frequencies based on the type of bond and atomic masses involved.
- Different types of vibrations include stretching, bending, scissoring, rocking, and twisting.
Absorption Bands:
- Each type of bond absorbs radiation at characteristic wavelengths, resulting in unique absorption bands.
Working Principle of FTIR
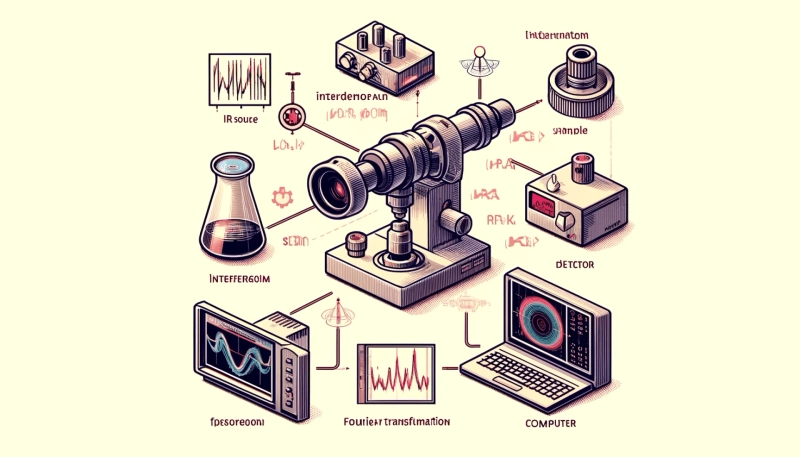
Interferometer:
- An interferometer splits an IR beam into two paths: one reflecting off a stationary mirror and the other off a moving mirror.
- When recombined, the beams produce an interference pattern based on the optical path difference.
Fourier Transformation:
- The raw interferogram data from the interferometer is converted into an IR spectrum through Fourier transformation.
Detector:
- The IR radiation passing through the sample is measured by a detector, providing the absorption or transmittance spectrum.
Schematic Representation of FTIR Spectroscopy
IR Source → Interferometer → Sample → Detector → ComputerAdvantages of FTIR
- Speed: Simultaneous measurement of all frequencies (multiplex advantage)
- Sensitivity: High signal-to-noise ratio
- Accuracy: High wavelength precision
- Versatility: Suitable for solids, liquids, and gases
Applications of FTIR
1. Chemical Identification and Characterization:
- Identification of unknown compounds by matching spectra to reference libraries.
- Characterization of functional groups and molecular structures.
2. Quality Control in Manufacturing:
- Monitoring raw materials and final products for purity.
- Detection of contaminants or degradation products.
3. Polymer Analysis:
- Understanding polymer composition and degradation.
- Identifying additives, fillers, and plasticizers.
4. Environmental Analysis:
- Detection of pollutants and hazardous substances in air, water, and soil.
- Identification of microplastics.
5. Forensic Analysis:
- Analysis of fibers, paints, and other trace evidence.
- Detection of explosives and narcotics.
Interpreting FTIR Spectra
Key Steps:
Baseline Correction:
- Correct for any background interference to improve spectral clarity.
Identify Absorption Bands:
- Locate characteristic peaks (wavelengths or wavenumbers).
Functional Group Identification:
- Match peaks to known functional groups using reference tables.
Quantitative Analysis:
- Determine the concentration of components based on peak intensity.
Common Functional Groups and Corresponding Peaks
- Hydroxyl (OH): 3200–3600 cm<sup>-1</sup> (broad)
- Carbonyl (C=O): 1650–1750 cm<sup>-1</sup>
- Aromatic (C-H stretch): 3030 cm<sup>-1</sup>
- Alkyl (C-H stretch): 2850–2960 cm<sup>-1</sup>
- Nitro (NO<sub>2</sub>): 1515 cm<sup>-1</sup> and 1345 cm<sup>-1</sup>
Conclusion
FTIR is a versatile and indispensable tool for material identification and analysis. Its ability to provide rapid, accurate, and detailed information about the molecular structure makes it invaluable across scientific disciplines. Understanding how to interpret FTIR spectra opens up a world of possibilities in quality control, research, and forensic investigations.
For more information about Fourier transform infrared spectroscopy lab in chennai, Fourier transform infrared spectroscopy