The demand for high quality and efficient medical devices, driven by a growing geriatric population and increasing incidence of various chronic clinical conditions, is on the rise. On the other hand, in order to ensure the safety and efficacy of healthcare products, regulatory authorities have increased the stringency of the review process related to the approval of such products. Consequently, the need to conduct adequate clinical studies has increased. However, owing to the complexities associated with clinical research, stakeholders in this domain have demonstrated a preference to outsource such operations. Currently, the medical device CRO industry is characterized by the presence of several established companies, as well as new players. In the coming years, we believe that the demand for contract research service providers is likely to increase significantly. This chapter provides a critical overview of the medical devices CRO market, highlighting the various strengths and opportunities, and the existing weaknesses and likely threats to the market’s evolution.
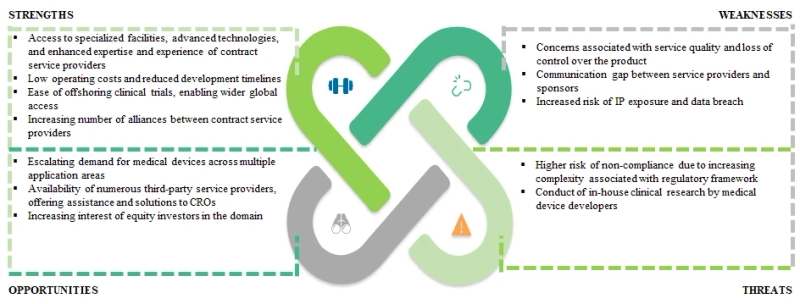
Figure 15.1 provides a pictorial representation of the key factors that are likely to impact the medical device CRO market, under a SWOT framework.
Figure 15.1 Medical Device CRO Market: SWOT Analysis
Source: Roots Analysis