Antigens are toxins or other foreign objects that induce immune system response. The immune system’s response is the production of Y-shaped proteins known as antibodies. These proteins are also known as immunoglobulins (Ig).
Antibodies are vital tools in research for identification, quantification, and isolation of specific proteins. Their ability to bind to specific antigens enables researchers to study disease mechanism, validate drug targets, and develop new diagnostic and therapeutic strategies.
This binding to specific antigens with high affinity and specificity makes antibodies useful in techniques like ELISA, Western blotting, immunohistochemistry and flow cytometry.
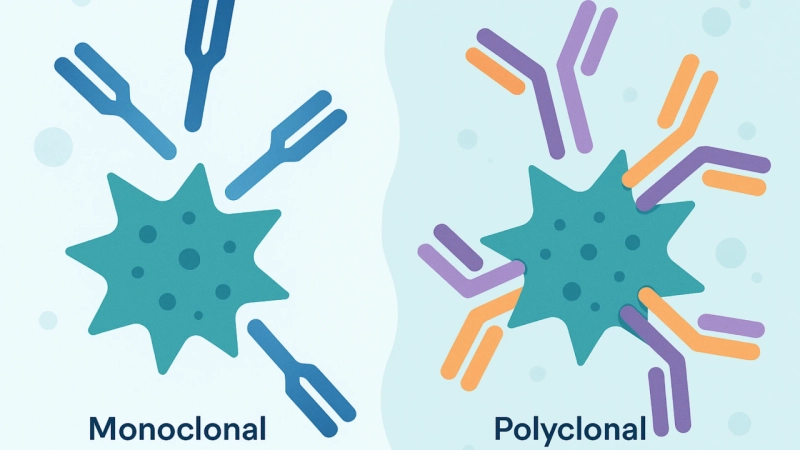
Monoclonal Antibodies (mAbs)
These antibodies are highly specific antibodies produced by identical immune cells. These antibodies descend from a single B-cell clone. Monoclonal antibodies bind to only one specific site on an antigen.
Polyclonal Antibodies (pAbs)
Produced by different B-cell clones, these antibodies are a heterogeneous mixture of antibodies. Polyclonal antibodies (pAbs) bind to multiple sites on the antigen.
This article aims to provide a clear and balanced comparison of monoclonal antibodies (mAbs) and Polyclonal antibodies (pAbs) to help researchers select the optimal antibody type for their experiments.
Production Methods & Core Characteristics
Polyclonal Antibody Production
Polyclonal antibody production includes four critical steps:
- Antigen preparation
- Animal immunization
- Antibody purification
- Quality control
Antigen preparation
In the antigen preparation step, the target antigen quality is paramount for antibody specificity and effectiveness. This is especially important when the antibodies are aimed to bind to a conformational epitope. Even if the impurity is less than 1% of the sample, it can be immunodominant, which means the immune system of the animal will react much more strongly to the impurity than the target antigen. Therefore, antigen purity is crucial.
Animal immunization
The choice of the right animal depends on the following factors:
- The desired amount of antiserum (serum that contains antibody)
- The evolutionary gap between the source of the antigen and the animal
A greater evolutionary distance induces a stronger immune response. Adjuvants are used to boost immune response in the animal. Commonly used animals include rabbits and goats. Rabbits have quite different genetics from common research targets (human and mice). Rabbits also provide a good amount of antibody-rich blood.
Antibody purification
The antiserum is a complex mixture that does not just contain the desired antibodies but also contains non-specific antibodies, albumin, clotting factors, and other undesired proteins. Purification is essential to reduce background noise and ensure reliable results.
Affinity chromatography is the most common method for polyclonal antibodies purification. In this method, the antigen used for immunization is attached onto a chromatography column matrix.
The antibodies specific to the attached antigen bind to the antigen itself. The unwanted proteins simply pass through.
Next, these bound antibodies are eluted from the antigens using salt concentration or change in pH. The result is a highly enriched and purified antibody solution.
Quality control
Research applications have rigorous standards. Specificity, sensitivity, and overall functionality of the antibody are crucial for reliable experimental results.
Specificity testing confirms that the antibody binds to the intended target antigen only and without cross-reaction with other proteins. Western blotting is an important test that verifies if the antibody correctly identifies a protein band of the right size. Western blotting also confirms that the antibody does not react with a sample that does not contain the desired protein.
Titer determination is used to measure the antibody’s functional concentration. High titer means less antibody is required for reliable results in the experiment. ELISA is the standard method used to determine titer. Functional assays confirm that the antibody works as expected in the intended application.
Methods like SDS-PAGE (Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis) are used for purity assessment.
Monoclonal Antibody Production
Difficult cell fusion, extensive screening, and careful maintenance of these living cells make monoclonal antibody production more complex and technically demanding. However, the produced antibodies are highly-specific and perform consistently. Monoclonal antibody production uses Hybridoma technology.
The production process involves the following six steps:
- Immunization
- Fusion of B Cells
- Selection of Hybridoma Cells
- Screening and Cloning
- Antibody Production and Purification
- Quality Control
Immunization
The process begins with the priming immunization, which is the introduction of the antigen into a small animal, typically a mouse or rat. Priming immunization aims to initiate immune response to produce B cells that generate antibodies against the introduced antigen. Next, booster injections are administered over several weeks to induce a strong and sustained immune response to ensure high production of antibody-producing B cells and promote affinity maturation. These highly-responsive B cells are essential for the entire production process.
Fusion of B Cells
B cells are harvested from the immunized animal, especially from the spleen as B cells from the spleen are rich in antibody-producing cells. However, B cells have a limited lifespan of a few days or weeks. This is the reason why these cells are fused with myeloma cells. Myeloma cells can divide indefinitely without producing their own antibodies. This fusion produces hybridoma cells which have the B cell’s ability to produce specific antibodies and the myeloma cell's immortality.
Selection of Hybridoma Cells
The cell mixture obtained after the fusion contains the following three types of cells:
- Unfused B cells
- Unfused myeloma cells
- Hybridoma cells
The cell mixture is grown in Hypoxanthine-Aminopterin-Thymidine medium for the isolation of only the hybridoma cells and to prevent the growth of unfused B cells and unfused myeloma cells.
Unfused B cells have a limited lifespan, and unfused myeloma cells lack a specific enzyme pathway crucial for survival in HAT medium.
Screening and Cloning
Identifying specific hybridoma clones producing the desired antibody is a challenge. ELISA (Enzyme-Linked Immunosorbent Assay) is the commonly used screening technique that tests supernatants from individual hybridoma cultures for their ability to bind specifically to the target antigen.
In cloning, hybridoma cells are diluted to a very low concentration, creating a highly dilute suspension which is distributed to individual wells. This ensures that each culture originates from a single hybridoma cell. Each hybridoma cell multiplies to form genetically identical cells. This group of cells is called a clone.
Screening and cloning ensures that the specific clone produces identical antibodies that bind to the same single binding site.
Antibody Production and Purification
A stable, antibody-producing hybridoma clone is then grown in large amounts to produce antibodies. This can be achieved in the following two ways:
In Vitro Production
In this method, hybridoma cells grow and secrete antibodies in large bioreactors or cell culture flasks containing the culture medium.
In Vivo Production
In this method, hybridoma cells are injected into the peritoneal cavity of mice, where they grow as tumors, secreting antibody-rich fluid in large quantities.
However, in vitro production is preferred due to ethical concerns.
Techniques like Protein A/G affinity chromatography are used to purify the monoclonal antibodies from the culture medium.
Quality Control
Just like polyclonal antibodies, the final monoclonal antibodies also undergo rigorous quality control. Specificity and purity testing using techniques like Western blot, ELISA, and SDS-PAGE ensure that the antibody is contamination-free and binds only to the intended target.
Titer determination measures the functional concentration of the antibody and functional assays validate the performance of the antibody in the intended applications. Monoclonal antibody quality control also ensures batch-to-batch consistency.
Monoclonal Antibodies (mAbs) Vs Polyclonal Antibodies (pAbs): Comparative Analysis
Specificity & Cross-reactivity
Monoclonal Antibodies (mAbs)
These antibodies exhibiting high specificity bind exclusively to a single binding site (epitope) on an antigen. As a result, non-specific interaction is minimal and cross-reactivity is reduced significantly.
Monoclonal antibodies (mAbs) are ideal for detecting subtle differences between closely related targets.
Polyclonal Antibodies (pAbs)
These antibodies recognize multiple binding sites on a single antigen which helps in detecting low-abundance targets. However, potentially high cross-reactivity can lead to higher background signal or false positives in some assays.
Consistency & Reproducibility
Monoclonal Antibodies (mAbs)
Monoclonal antibodies (mAbs) offer excellent batch-to-batch consistency and reproducibility ideal for standardized assays, diagnostics, and therapeutics demanding high consistency.
Polyclonal Antibodies (pAbs)
As polyclonal antibodies (pAbs) are derived from different immunized animals, the variability in the mixture of antibodies can lead to inconsistent specificity, affinity, and performance between batches.
Production & Cost
Monoclonal Antibodies (mAbs)
Complex and technically demanding production of monoclonal antibodies (mAbs) requires high initial investment. However, after establishing a stable hybridoma clone, cost per unit for large-scale production is significantly reduced. The stable hybridoma clone can produce large quantities of identical antibodies indefinitely.
Polyclonal Antibodies (pAbs)
A simple process and short production timeline means lower initial cost. However, the quantity of the obtained antibodies depends on the lifespan and blood volume of the immunized animal. Continued supply of these antibodies requires immunization of a new animal which leads to batch variability.
Applications & Advantages
Monoclonal Antibodies (mAbs)
As monoclonal antibodies bind to a single specific epitope, they are ideal for applications that require highly specific detection and minimal background noise. These applications include clinical diagnostics such as highly accurate pregnancy tests, specific cancer marker detection, infectious disease identification and more.
- Many modern biologics and targeted therapies for conditions like cancer and autoimmune diseases use monoclonal antibodies to modulate or neutralize specific targets with precision.
- Monoclonal antibodies (mAbs) are used in ELISA and other quantitative assays requiring accurate and consistent measurement.
- As monoclonal antibodies precisely bind to specific epitope, they are useful in the studies of specific protein isoforms, phosphorylation states, or conformational changes.
- The reproducibility and batch-to-batch consistency of monoclonal antibodies make them ideal for long-term studies, clinical trials, and regulatory approval.
Polyclonal Antibodies (pAbs)
As polyclonal antibodies can recognize multiple epitopes, they can effectively detect targets that may be
- Denatured or degraded
- Present in very low abundance
The ability to bind to multiple sites on an antigen can amplify signal and offer higher overall sensitivity than mAbs.
When extreme specificity is not a concern, polyclonal antibodies (pAbs) are a good choice for initial screening, preliminary studies, or general protein detection.
These antibodies are also efficient for immunoprecipitation (IP) and immunodepletion.
Limitations & Disadvantages
Monoclonal Antibodies (mAbs)
Sensitivity to epitope changes
Monoclonal antibodies (mAbs) may not bind effectively if the epitope is masked, denatured, or altered due to mutations or post-translational modifications.
High upfront cost and time
The initial development is expensive and time-consuming due to the complex and technically demanding nature of hybridoma technology.
Limited signal amplification
Binding to only one epitope can sometimes lead to lower signal intensity.
Polyclonal Antibodies (pAbs)
Batch-to-batch variability
Polyclonal antibodies (pAbs) require extensive re-validation. Inconsistent performance between different lots can complicate long-term studies.
Higher background and cross-reactivity
Binding to non-target proteins can result in higher background noise in assays.
Finite supply
Continuous supply of polyclonal antibodies (pAbs) requires new animal immunizations.
Conclusion
Whether you are looking for polyclonal antibodies (pAbs) or monoclonal antibodies (mAbs), purchase from reliable suppliers such as AAA Biotech. Consider the unique characteristics, advantages, and disadvantages to make an informed decision.