Competitive benchmark analysis allows the companies to compare their existing capabilities within their respective peer groups (based on company size and location of headquarters) and thereby, identify ways to gain a competitive edge in the industry. In addition, this analysis can be used by companies to spot potential improvement areas by identifying gaps between their existing capabilities and industry best practices.
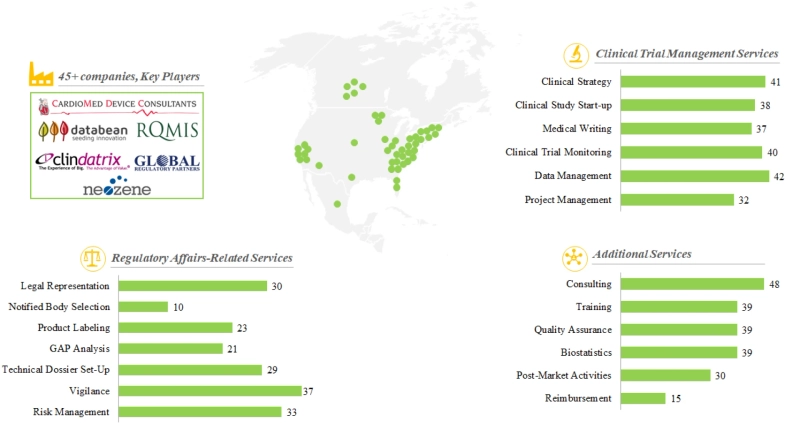
The following peer group includes small medical device CROs that are based in North America. According to our selection criteria, there are 48 companies in this peer group. Figure 8.2 presents a summary of the existing benchmarks (in terms of CRO capabilities and services offered) within this peer group, as inferred from our research.
Figure 8.2 Competitive Benchmarking: North America, Peer Group I
Note 1: The position of the dots in the representation highlights the locations of headquarters of the companies considered in the analysis
Note 2: Companies offering more than one type of clinical trial management / regulatory affairs-related / additional services have been counted multiple times in this representation
Note 3: Key players in a peer group are companies that claim to provide 16+ contract research services
Source: Roots Analysis
The charts present distribution of companies in peer group I across the following parameters:
Clinical trial management services: We observed that 60% companies in this peer group offer more than three clinical trial management services. Further, it is worth highlighting that more than 40% companies in this peer group claim to offer all six clinical trial management services for medical devices.
Regulatory affairs-related services: We observed that 50% companies in this peer group offer vigilance and risk management services to its customers. Examples of companies that offer more than five regulatory services include (in alphabetical order) AJW Technology Consultants, ClinDatrix, Global Regulatory Partners, Morley Research Consortium, Neozene and PRC Clinical.
Additional services: We observed that all the companies in this peer group offer consulting services, while over 55% companies offer more than three additional services for medical devices. Examples of companies that offer maximum number of additional services include (in alphabetical order) CardioMed Device Consultants, Clinical Device Group, Databean, Promedica International, RQMIS, The CRO Group and Vantage BioTrials.