The number and geographical distribution of clinical trials are important indicators of both the therapeutic viability and future potential of innovative pharmacological interventions. These trials enable CDMOs / CMOs to estimate the clinical demand of peptide therapeutics across different geographies, therapeutic areas and trial phase. Moreover, the geographical distribution is a direct indicator of the various markets that are conducting trials or enrolling patients for clinical studies. Further, as more product candidates are approved by regulatory authorities across the globe, the number of clinical trials are also anticipated to increase.
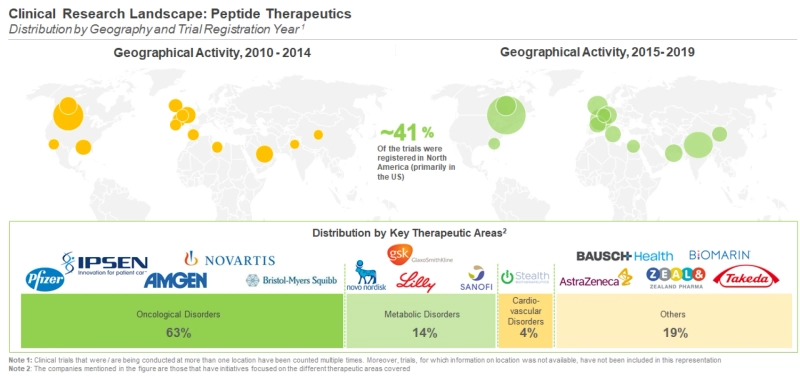
Majority of the completed / ongoing / planned trials for peptide therapeutics are centered in North America. Further, within this region, the US (623) emerged as the most prominent hub for conducting clinical trials for peptide therapeutics. Within Europe, the maximum number of trials (125) were reported to be / have been conducted in Germany. In Asia Pacific, the maximum number of peptide therapeutics focused trials were registered in Georgia (114), followed by India (96).
Majority (330) of the active clinical trials are being conducted in North America, wherein more than 40% of the trials are currently in phase II. Similarly, in Europe, majority (46%) of the active trials are in phase II. Whereas, in Asia Pacific and rest of the world, most of the active clinical trials are in phase III. This indicates that there is a higher clinical demand in North America and Europe, as compared to Asia Pacific, where a significant number of active trials are inching towards completion.
The maximum number of patients were enrolled in studies being conducted in North America. Within this region, most of the patients were enrolled in the US (70,384). In Europe, most of the patients were enrolled for peptide therapeutics-based studies in the UK (8,665). Within Asia Pacific, majority of the patients were enrolled in studies based in China (15,883). The majority (70%) of the patients were enrolled in phase III clinical trials in North America.
Majority (63%) of the active trials are evaluating peptide therapeutics intended for the treatment of oncological disorders, primarily multiple myeloma and prostate cancer. This is followed by studies evaluating peptides targeting metabolic disorders (14%) and cardiovascular disorders (4%). Other prominent therapeutic areas include genetic disorders, neurological disorders and gastroenterological disorders. Similarly, majority (60%) of the patients were enrolled for trials evaluating peptide therapeutics intended for the treatment of oncological disorders, primarily for multiple myeloma and prostate cancer. This is followed by patients enrolled in trials for peptides targeting metabolic disorders (22%) and cardiovascular disorders (4%).