In the medical device industry, the margin for error is razor-thin. Regulatory agencies like the FDA expect manufacturers to ensure not only that their devices are safe and effective, but also that the data supporting them is trustworthy, consistent, and compliant. That’s where a rock-solid data integrity program and robust computer software assurance strategies become mission-critical.
At Compliance Gurus, we help medical device companies navigate these complex requirements — from CSV audit readiness to FDA remediation — so they can stay compliant, agile, and market-ready.
Why Data Integrity Is the Backbone of Compliance
Data integrity isn’t just about avoiding errors; it’s about ensuring data is complete, consistent, accurate, and available throughout its lifecycle. In regulated industries, the ALCOA+ principles — Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available — guide how data should be managed.

A rock-solid data integrity program addresses:
- People – Training staff on proper data handling and documentation.
- Processes – Defining SOPs to capture, store, and review data effectively.
- Technology – Implementing systems with audit trails, security controls, and validation.
Without this foundation, even the most advanced software assurance strategies can fail under regulatory scrutiny.
CSV Audit Readiness: Preparing for Success
CSV (Computer System Validation) is the traditional method used to confirm that software meets its intended purpose and regulatory requirements. Being CSV audit-ready means:
- Your validation documentation is clear, complete, and up-to-date.
- Risk assessments are thorough and traceable.
- Test scripts are accurate and linked to requirements.
- Change controls and deviations are well-documented.

FDA auditors often focus on gaps in traceability and missing documentation. At Compliance Gurus, we help you maintain CSV audit readiness through proactive reviews, gap assessments, and mock inspections — so when the real audit comes, you’re already ahead of the game.
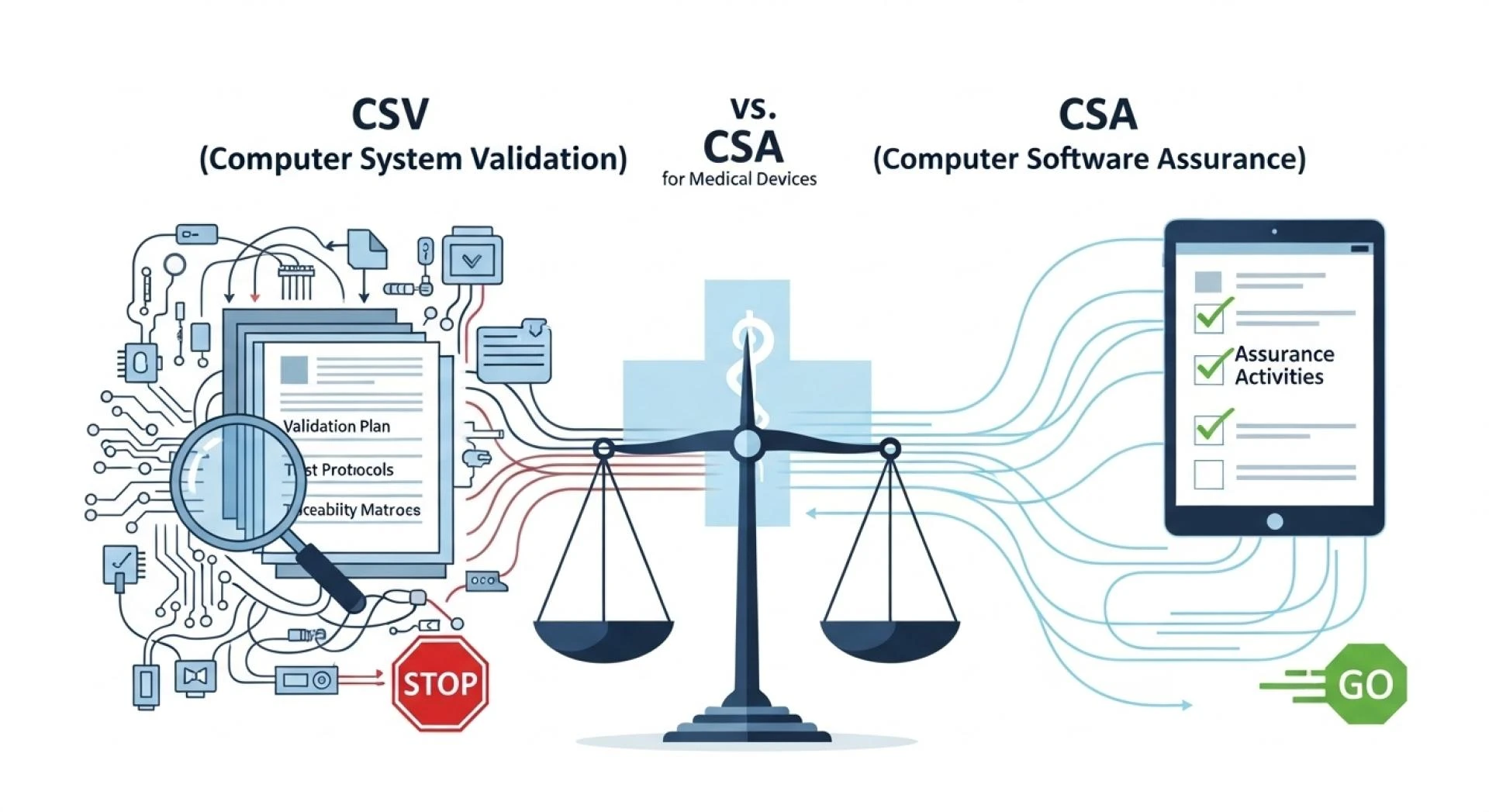
The Difference Between CSA and CSV
Many companies are asking about the difference between CSA and CSV. Here’s the breakdown:
- CSV (Computer System Validation)
- Follows a documentation-heavy approach, focusing on extensive testing and paper-based evidence to demonstrate compliance.
- CSA (Computer Software Assurance)
- A modern, risk-based approach endorsed by the FDA. It emphasizes critical thinking, targeted testing based on risk, and leveraging automation where possible.

When comparing CSV vs CSA for medical devices, CSA is often more efficient because it prioritizes testing that impacts patient safety and product quality, rather than spending equal effort on low-risk features. However, transitioning to CSA requires a shift in mindset, processes, and sometimes even technology.
Data Integrity Programs and Assessments
Even with the best intentions, companies can fall short if they don’t periodically assess their compliance health. Data integrity programs and assessments help identify:
- Weak spots in SOPs and workflows.
- Gaps in training or awareness.
- Vulnerabilities in IT systems and security.
- Inconsistencies in how data is captured, stored, and retrieved.

Our team conducts both internal readiness assessments and third-party compliance audits, giving you a clear roadmap to strengthen your systems before a regulator points out the flaws.
Cloud-Based Software Assurance: The Modern Advantage
Medical device companies are increasingly shifting to cloud-hosted platforms for speed, scalability, and collaboration. But with this shift comes the need for cloud-based software assurance.

Key considerations include:
- Vendor qualification and risk assessment.
- Ensuring compliance with data residency and security requirements.
- Continuous monitoring of system performance and access controls.
- Leveraging CSA principles for validation in a cloud environment.
With cloud adoption growing, the FDA and other agencies expect companies to demonstrate that cloud-based systems are just as secure and reliable as on-premises solutions.
FDA Remediation: Turning Setbacks into Opportunities
If your organization has received an FDA warning letter, 483 observation, or other regulatory finding, FDA remediation becomes a top priority. This process involves:
- Root Cause Analysis – Understanding not just what went wrong, but why.
- Corrective Actions – Implementing fixes that address both the symptom and the cause.
- Preventive Actions – Ensuring the same issue doesn’t recur.
- Documentation – Providing evidence that remediation steps are effective.

While remediation can be stressful, it’s also a chance to modernize systems, strengthen processes, and demonstrate commitment to quality.
Data Integrity Services by Compliance Gurus
Our data integrity services are designed for end-to-end compliance support, including:
- Program design and implementation.
- Validation and assurance services.
- Audit preparation and defense.
- Gap analysis and remediation planning.
- Ongoing compliance monitoring.

By partnering with Compliance Gurus, medical device companies gain a team of experts who understand both the letter and the spirit of regulatory requirements.
The Future of CSV vs CSA for Medical Devices
As the FDA continues to advocate for CSA and other risk-based approaches, the medical device industry will need to adapt. But CSV isn’t disappearing — it will still be relevant in certain contexts, particularly for legacy systems and markets outside the U.S. The key will be balancing efficiency, risk management, and compliance.
Forward-looking companies will:
- Integrate CSA principles into their validation processes.
- Invest in digital tools that streamline testing and documentation.
- Regularly update their data integrity programs to address emerging threats.
- Use cloud-based software assurance to support global operations.
Final Thoughts
Whether you’re preparing for an audit, transitioning from CSV to CSA, or responding to an FDA finding, the principles remain the same: strong data integrity, effective software assurance, and proactive compliance management.
At Compliance Gurus, we bring expertise, precision, and practical solutions to every engagement. With our support, your organization can confidently meet regulatory requirements, safeguard patient safety, and maintain the trust of customers and regulators alike.
Call to Action:
Ready to strengthen your compliance framework? Contact Compliance Gurus today for a tailored consultation on data integrity services, software assurance, and FDA remediation solutions for medical devices.