A new study by researchers at North Carolina State University has revealed how to extend the technology commonly used to manufacture single metal nanoparticles to the manufacture of nanoparticles composed of two metals with adjustable properties. This study also provides some insights into the optical properties of nanoparticles.
Adjusting the optical properties of nanoparticles is of great interest in applications such as safety technology and improving the efficiency of chemical reactions (with a variety of industrial and environmental applications).
Researchers have created core/shell nanoparticles with gold core and silver shell, and alloy nanoparticles mixed with gold and silver. The researchers also described the optical properties of these nanoparticles. "Silver and gold have unique optical properties, which are caused by the specific interaction between them and the electric field of light," said Dr. Joe Tracy, assistant professor of materials science and engineering at North Carolina State University, co-author of a paper describing the study. "By controlling the proportion of metals and whether nanoparticles have core/shell or alloy structure, we can control the optical properties of nanoparticles."
Researchers used a technique called "digestion maturation" to synthesize nanoparticles. This technology has been used to manufacture single metal particles for about ten years, but the research on manufacturing core/shell and alloy nanoparticles using mature digestion technology is limited. However, the comprehensiveness of this study may make it more common.
"This study and the related work of others show that digestion and maturation is a feasible method for manufacturing multicomponent metal nanoparticles. We use gold and silver, but the same principle may also apply to other metals," Tracy said. "Our detailed evaluation of this synthesis method should help other researchers explore other kinds of binary metal nanoparticles."
Digestion and maturation depend on the use of ligands, which are small organic molecules, some of which are directly bound to metals. Ligands are usually fixed on the metal core of nanoparticles to prevent nanoparticles from gathering together, so that nanoparticles are suspended in solution. When the ligand can transfer metal atoms from the core of one nanoparticle to the core of another nanoparticle, digestion and maturation will occur, so that the size distribution between nanoparticles is more uniform.
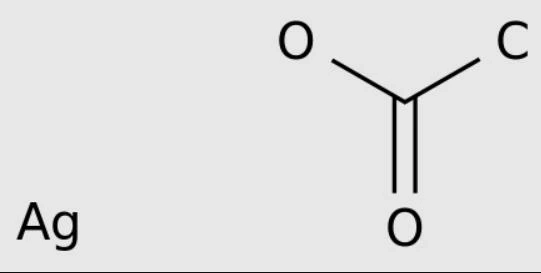
The researchers used the mature digestion technology to produce a solution of gold nanoparticles of similar size. When they introduced silver acetate into the solution, the ligand transported silver atoms to the surface of gold nanoparticles, resulting in nanoparticles with gold core and silver shell.
The researchers then transferred the nanoparticles to a second solution containing different ligands. Heating the second solution to 250 degrees Celsius causes the metals to diffuse to each other, resulting in nanoparticles made of gold and silver alloy.
The researchers also produced gold silver alloy nanoparticles by skipping the step of forming the shell, introducing silver acetate into the second solution and raising the temperature to 250 ℃. The advantage of this "shortcut" method is that it simplifies the control of the ratio of gold to silver in the alloy.