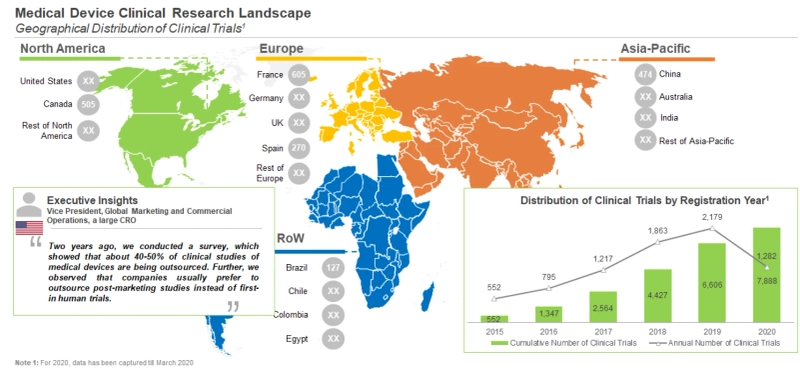
The number and geographical distribution of clinical trials are important indicators of both therapeutic viability and future potential of innovative pharmacological interventions and medical devices. Pharmaceutical / medical device companies are likely to invest in conducting extensive clinical studies on only those devices that are likely to translate into commercial success stories. Moreover, the geographical distribution is a direct indicator of the various markets that are conducting trials or enrolling patients for clinical studies. Further, as more devices are approved by regulatory authorities across the globe, the number of clinical trials is anticipated to increase.
In case of medical devices, the requirement of conducting clinical trials depends on the level of risk associated with the use of the device. For instance, most of the regulatory authorities across the globe do not publish guidelines for medical devices (such as adhesive bandages) that pose negligible risk to human subjects, and therefore do not require a clinical trial. However, devices that pose high risk, such as drug-eluting stents or materials for hip replacement, may necessitate a clinical trial. It is important to highlight that the trial design for drugs and medical devices varies considerably. Unlike clinical trials evaluating drugs, studies for medical device are usually evaluated in two phases, pilot phase (comprising of 10-30 diseased subjects) and pivotal phase (comprising of 150-300 diseased subjects).[1]
In terms of therapeutic area, majority of the clinical trials are evaluating medical devices intended for the treatment of CNS disorders, primarily stroke, Parkinson's disease and Schizophrenia. This is followed by studies evaluating medical devices targeting cardiovascular disorders and oncological disorders. It is worth highlighting that the number of medical devices focused clinical trials targeting CNS disorders increased at a CAGR of 48%, between 2015 and 2019. However, the CAGR for cardiovascular and oncological disorders during the same period was calculated to be 17% and 21%, respectively. Some of the prominent indications for which medical devices are being evaluated in clinical studies include (in decreasing order of number of clinical trials) prostate cancer, coronary artery disease, heart failure, breast cancer, knee osteoarthritis and peripheral arterial disease. In terms of patient enrolment, majority of the patients were enrolled for trials evaluating medical devices intended for the treatment of ophthalmic disorders, primarily for blindness, visual impairment, refractive error, cataract and high myopia. This is followed by patients enrolled in trials targeting cardiovascular disorders and oncological disorders.