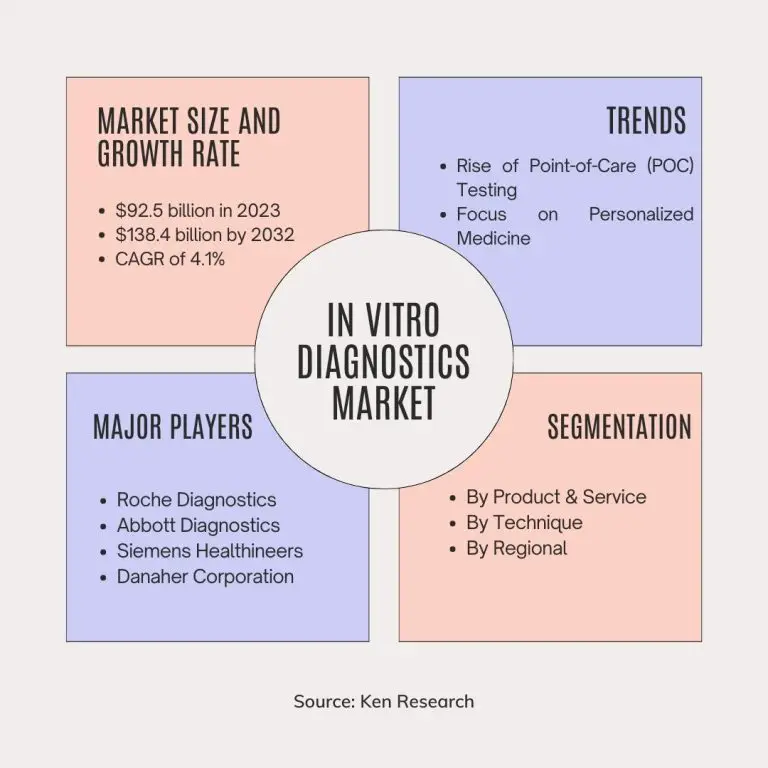
The In Vitro Diagnostics Market was valued at $92.5 billion in 2023 and is projected to reach a staggering $138.4 billion by 2032. This growth reflects a compound annual growth rate (CAGR) of 4.1% from 2023 to 2032. The continuous demand for advanced diagnostic tools underscores the vital role of IVD in enhancing patient care and healthcare outcomes.
Trends in the In Vitro Diagnostics Market
The In Vitro Diagnostics (IVD) market is undergoing exciting advancements and is expected to see significant growth in the coming years
- Rise of Point-of-Care (POC) Testing: There's a growing demand for rapid and convenient diagnostic tests outside traditional laboratory settings. POC tests empower patients with self-testing options and expedite treatment decisions in clinics and physician offices.
- Focus on Personalized Medicine: The IVD market is increasingly driven by personalized medicine, tailoring diagnostics to individual patients' genetic makeup and health conditions. This trend is fueling the development of companion diagnostics that guide treatment decisions for specific drugs.
- Technological Advancements: The IVD industry is embracing new technologies like molecular diagnostics, automation, and artificial intelligence (AI). Molecular diagnostics offer faster and more accurate test results, while automation improves efficiency and reduces errors. AI-powered tools are being incorporated for data analysis, image recognition in diagnostics, and even automation of certain lab processes.
- Growing Importance of Near-Patient Testing: Similar to POC testing, near-patient testing allows for quicker diagnoses closer to the patient, often in settings like emergency departments or surgical suites. This trend is particularly relevant for tests guiding critical care decisions.
Read Also:- The In Vitro Diagnostics Industry Size, Market Growth with Trends and Top Players
Major Players in the In Vitro Diagnostics Sector
The In Vitro Diagnostics (IVD) industry is dominated by a few established players who hold a significant share due to their vast product portfolios and global reach.
- Roche Diagnostics (Switzerland): A dominant player offering a comprehensive range of IVD tests across various disease areas.
- Abbott Diagnostics (US): Another major force, well-known for its blood tests, immunoassays, and instruments for various diagnostic applications.
- Siemens Healthineers (Germany): A leading provider not only in IVD tests but also in medical imaging equipment, offering a holistic diagnostic solution.
- Danaher Corporation (US): A diversified company with a large diagnostics segment, including well-regarded companies like Beckman Coulter in the IVD space.
- Johnson & Johnson Medical Devices and Diagnostics (US): Offers a broad portfolio of diagnostic products, encompassing blood tests, medical imaging equipment, and other IVD solutions.
- BioMérieux (France): A world leader in in vitro diagnostics, with a strong specialization in microbiology and infectious disease testing.
In Vitro Diagnostics Market Segmentation
The In Vitro Diagnostics market can be segmented by several key factors that provide insights into market trends and cater to specific needs within the industry. Here's a breakdown of some common segmentation approaches:
By Product & Service
- Reagents & Kits: These are the consumable elements of IVD tests, containing the chemicals and materials needed to perform the analysis on a sample. This segment holds a dominant market share due to the frequent use of test kits and ongoing demand for reagents.
- Instruments: Diagnostic instruments analyze the samples using various technologies. This segment encompasses a wide range of equipment, from complex analyzers for sophisticated tests to basic readers for simpler assays.
- Software & Services: This segment includes data management software for diagnostic instruments, interpretation tools for test results, and related IT services to maintain and optimize laboratory operations.
By Technique
- Immunoassays: A widely used technique that detects antigens or antibodies in a sample. Immunoassays are employed for various applications like infectious disease testing, hormone level testing, and bloodborne pathogen screening.
- Molecular Diagnostics: This technique analyzes a patient's DNA or RNA to identify abnormalities or pathogens. Molecular diagnostics play a crucial role in cancer diagnosis, genetic testing, and infectious disease testing with high specificity.
- Clinical Chemistry: Analyzes the biochemical makeup of bodily fluids like blood and urine to assess organ function and identify metabolic disorders. Common clinical chemistry tests include blood sugar testing, liver function tests, and kidney function tests.
- Hematology: Focuses on analyzing blood cells and components, providing information about blood production, clotting function, and potential blood disorders. Complete blood count (CBC) is a common hematology test.
- Microbiology: Identifies and characterizes microorganisms that cause infectious diseases. Microbiology techniques involve culturing microorganisms and employing various methods for identification.
By Regional
- North America: North America holds a significant share of the IVD market, driven by a well-established healthcare infrastructure and high prevalence of chronic diseases. The United States, in particular, is a major contributor to the market growth.
- Europe: Europe is another major market for IVD, with a strong focus on advanced diagnostics and personalized medicine. The region benefits from a robust healthcare system and regulatory support.
- Asia Pacific: The Asia Pacific region is experiencing rapid growth in the IVD market due to increasing healthcare expenditure, rising awareness, and expanding access to healthcare services.
- Middle East and Africa: The Middle East and Africa are emerging markets for IVD, with growing investments in healthcare infrastructure and increasing prevalence of infectious diseases.
- Latin America: Latin America presents growth opportunities for the IVD market, driven by increasing healthcare investments and rising awareness about early diagnosis and disease management.
In Vitro Diagnostics Industry Challenges
The In Vitro Diagnostics (IVD) Sector is a dynamic field with promising growth potential, but it also faces certain challenges.
- Budget Constraints: Healthcare systems around the world face tight budgets, which can limit the adoption of new and expensive IVD technologies. Balancing the cost-effectiveness of these tests with their clinical benefits is crucial for wider acceptance.
- Regulatory Stringency: The IVD industry is heavily regulated to ensure the accuracy and safety of diagnostic tests. Strict regulatory processes can be time-consuming and expensive for manufacturers, potentially hindering innovation and delaying the launch of new products.
- Cybersecurity Threats: As IVD instruments and software become increasingly digital, the risk of cyberattacks on healthcare data rises. Ensuring robust cybersecurity measures is essential to protect patient information and maintain trust in diagnostic results.
- Reimbursement Policies: Reimbursement policies by insurance companies can significantly impact the adoption of IVD tests. If the tests are not adequately reimbursed, healthcare providers may be hesitant to use them, limiting their accessibility to patients.
Read Also:- The $131 Billion In Vitro Diagnostics Market, Industry Segments and Trends
Conclusion
The In Vitro Diagnostics market is poised for significant growth, driven by the increasing prevalence of chronic and infectious diseases, advancements in technology, and the rising adoption of personalized medicine. Despite challenges such as regulatory compliance and reimbursement procedures, the market presents numerous opportunities for innovation and expansion. As the industry continues to evolve, staying abreast of trends and developments will be crucial for stakeholders to capitalize on the potential of the IVD market.