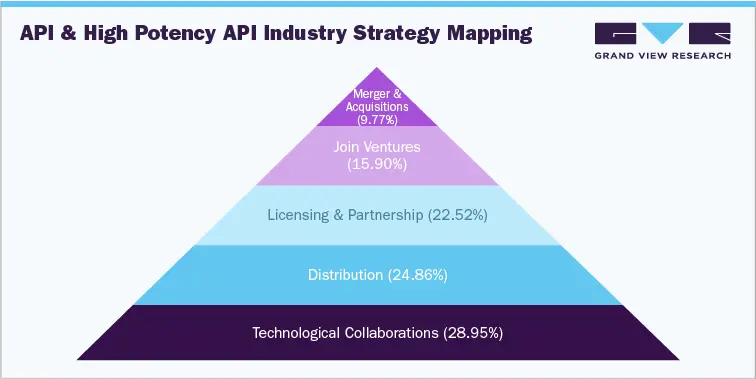
Global API and High Potency API industry databook is a collection of market sizing & forecasts insights, regulatory & technology framework, pricing intelligence, competitive benchmarking analyses, macro-environmental analyses studies. Within the purview of the database, such information is systematically analyzed and provided in the form of summary presentations and detailed outlook reports on individual areas of research.The following data points will be included in the final product offering in two reports and one sector report overview.
The global active pharmaceutical ingredients market was valued at USD 209.71 billion in 2021 and is anticipated to witness growth at a rate of 5.9% over the forecast period. The global high potency API market was valued at USD 23.60 billion in 2021 and is anticipated to witness growth at a rate of 6.2% over the forecast period.
Active Pharmaceutical Ingredients Market Growth & Trends
The global active pharmaceutical ingredients market was valued at USD 209.71 billion in 2021 and is anticipated to witness growth at a rate of 5.9% over the forecast period. The Active Pharmaceutical Ingredients market is becoming more competitive and consumer needs are becoming highly focused toward efficacy & specificity of therapeutic agent raw material. Buyers tend to purchase materials matching their requirements and negotiate the price. Thus, buyers are highly involved in the purchasing process to ensure quality of material matches their requirement.
High capital is required for production of APIs because the process needs extremely systematic protocols, which leads to increase in outsourcing of various API production processes. Companies are looking to diversify their API suppliers and manufacturers to different locations instead of outsourcing it to just one manufacturer. For instance, in November 2022, Novo Nordisk spent USD 747 million for expanding its API manufacturing facility in Denmark, in November 2022, GE Healthcare invested USD 80 million for expanding its API manufacturing site in Norway. Companies that have been outsourcing API manufacturing to China to reduce costs are now aiming toward switching to other countries and are looking for backward integration. This is creating growth opportunities for other leading API-producing countries, such as India, to increase market shares in the changing market scenario.
Innovative Active Pharmaceutical Ingredients market dominated the market with a revenue share of 53.5% in 2021. Favorable regulations for R&D facilities and increase in funding are key factors driving the innovative Active Pharmaceutical Ingredients market. Owing to extensive research in this field, many novel innovative products are now in pipeline and are expected to be launched over the forecast period. New entrants in this segment are expected to drive the market. An example of a market player engaged in extensive R&D is Lonza, with more than 575 clinical development programs in 2018. Oncology is a leading domain for high-potency innovative API products due to need for low dosage and fewer adverse effects. Thus, highest percentage of HPAPIs is used in anticancer drugs. These APIs have also found application in hormonal imbalance, cardiovascular, musculoskeletal, and CNS drugs as well as in treatment of glaucoma.
Order your copy of Free Sample of “Active Pharmaceutical Ingredients and High Potency API Industry Data Book – Active Pharmaceutical Ingredients and High Potency Active Pharmaceutical Ingredients Market Size, Share, Trends Analysis, And Segment Forecasts, 2023 – 2030” Data Book, published by Grand View Research
High Potency Active Pharmaceutical Ingredients Market Growth & Trends
The global high potency API market was valued at USD 23.60 billion in 2021 and is anticipated to witness growth at a rate of 6.2% over the forecast period. HPAPIs are attracting generic manufacturers’ attention due to patent expiry of blockbuster drugs. Moreover, CDMOs are constantly coming up with lower Occupational Exposure Limits (OELs) for intermediates and materials, which ensures employee safety.
For addressing the unmet medical needs, companies are collaborating to develop novel High Potency Active Pharmaceutical Ingredients (HPAPIs). This allows firms to use their resources to aid the development of products and enhance their supply chain. For instance, in January 2022, Lonza, BioGeneration Ventures (BGV), and Forbion announced the extension of their collaboration to include development & production of small molecules (biologics). Lonza will provide customized services to BGV and Forbion’s large molecule biologic portfolio.
Captive high potencyAPI manufacturing market segment is anticipated to hold high revenue share. Outsourced market segment is driven by factors such as rising demand for biopharmaceuticals and growing cost of manufacturing these molecules in-house. Moreover, dual sourcing is becoming a key strategy and increasingly being adopted by these companies. HPAPI molecules are manufactured by some pharmaceutical companies at in-house manufacturing facilities. Pharmaceutical companies invest heavily to develop in-house capabilities and specialized requirements for toxicity containment. According to Japan Pharmaceutical Manufacturers Association, Japanese pharmaceutical companies have continuously increased their investments in R&D for discovery and development of novel drugs every year. These investments represent more than 17% of total net sales. These companies have also launched their own research funds to collaborate with outside researchers to develop new drugs. For example, a3 (a-cube) and TaNeDS launched by Astellas Pharma and Daiichi Sankyo, respectively.
Competitive Landscape
Key players operating in the Active Pharmaceutical Ingredients and High Potency API Industry are –
• Novo Nordisk
• Merck KGaA
• Novartis AG
• Aurobindo Pharma
• Piramal Pharma
• Teva Pharmaceutical Industries Ltd.
• AbbVie, Inc.
• BASF SE
• Boehringer Ingelheim International GmbH
• CordenPharma
• Merck & Co., Inc.
• Dr. Reddy’s Laboratories Ltd.
• Cipla, Inc.
• Bristol-Myers Squibb Company
• Sun Pharmaceutical Industries Ltd.
• Sanofi
• Pfizer, Inc.
• Viatris Inc.
• Albemarle Corporation
• Aurobindo Pharma