Kratom-Pharmacology, Clinical Implications and Deep Review
Kratom, or Mitragyna, is a tropical plant indigenous to Southeast Asia, with unique pharmacological properties. It is commonly consumed by preparing the leaves into decoction or tea, or by grinding them into a powder. Recent evidence has revealed that kratom has physiological effects similar to opioids, including pain relief and euphoria, as well as stimulant properties, which together raise potential concern for dependence and addiction. Moreover, growing evidence suggests that the prevalence of kratom use is increasing in many parts of the world, raising important considerations for healthcare providers. This manuscript will discuss the most current epidemiology, pharmacology, toxicity, and management related to kratom, while seeking to provide a contemporary perspective on the issue and its role in the greater context of the opioid epidemic.
Key Summary Points
Kratom (Mitragyna speciosa) is a botanical supplement with unique psychoactive properties.
The prevalence of kratom use appears to be increasing in Europe and North America, raising concerns for its possible development into a significant public health threat.
The body of scientific literature concerning kratom is expanding, but has not yet sufficiently characterized the nature and extent of the potential risks posed by kratom.
There is an increasing need for healthcare providers to be familiar with kratom and the management of patients who abuse it.
Introduction
Mitragyna speciose (Korth) is a tree-like herb consumed for its distinctive psychotropic properties [1]. Commonly known as “kratom”—a term referring to both the plant itself and the botanical products derived from its leaves—the M. speciosa tree is a tropical evergreen indigenous to the southeastern Asia-Pacific region, sharing close phylogeny with the coffee plant in the Rubiaceae family [2]. The consumption of kratom has been commonplace within this region for centuries, but has also recently gained popularity in the West [3, 4].
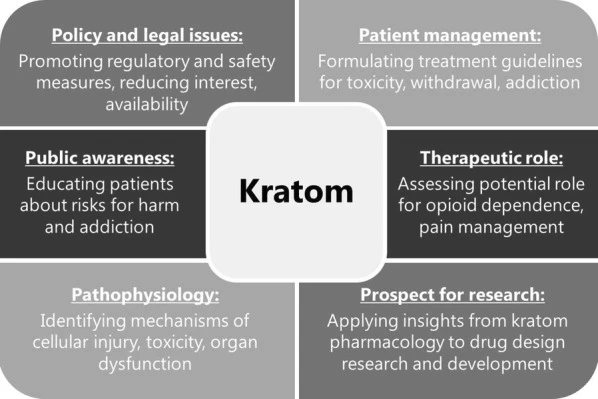
Kratom is primarily sought out for its stimulant and opioid-like properties, and may be used either for its perceived therapeutic effects or as a recreational drug. In either case, there is considerable uncertainty regarding the safety of ingesting kratom products. Consequently, it is important that healthcare providers be familiar with the subject, as it represents a growing public health concern. There are multiple aspects for the medical field to consider in addressing the problem of kratom, including reducing interest and accessibility, optimizing management of toxicity and dependence, and investigating its prospective use in research and therapeutics (Fig. 1)
Fig 1. Key considerations regarding kratom in the medical field. Figure source: ncbi.nlm.nih.gov
The purpose of this review is to provide an in-depth discussion of these points, framing them within the greater context of the opioid crisis at large. Specifically, the article seeks to address the current epidemiology, pharmacology, and toxicity associated with kratom. In addition, we provide a synopsis on the clinical management of kratom in order to assist caretakers as they address patients suffering from overdose, addiction, and withdrawal related to the drug. To achieve these objectives, we have conducted an extensive and detailed literature review of the subject, incorporating both preclinical studies and clinical case reports in order to provide a fuller perspective on the matter.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Kratom: Background, Prevalence, and Legal Status
Kratom use has been customary in countries such as Thailand, Malaysia, and Myanmar for several hundred years [5]. Depending on the specific region, kratom is alternatively known as ketum, biak–biak, ithang, or thom [6]. Although raw leaves can be chewed or smoked for the effects, more frequently the leaves are boiled in water to produce decoctions or teas, which contain multiple biologically active phytochemicals, accounting for its psychoactive properties [3, 7]. In addition to these more traditional methods of preparation, the leaves may be dried and processed into powders, capsules, and extracts, especially in western countries [8].
Historically, kratom has been used in traditional folk remedies for treating a range of ailments, for example, to mitigate symptoms of opiate addiction and withdrawal, or for weaning off dependence [9, 10]. It is also frequently used to relieve pain, produce euphoria, and stave off fatigue, especially among laborers in rural areas [11]. Its potential for dependence and addiction has long been apparent, and led to its categorization as a banned substance in both Malaysia and Thailand in the mid-twentieth century (of note, the Thai National Assembly has recently made it legal for medical purposes) [12, 13]. Nevertheless, the illicit use of kratom remains common; for instance, a survey conducted in Thailand in 2011 estimated the nationwide prevalence (lifetime) to be 2.9%, with nearly half of those admitting to daily kratom use, making it among the most commonly used illicit substance in the country [14].
In recent years, commercial preparations of kratom have become increasingly available in regions far beyond its local origins. Large-scale epidemiological studies evaluating the prevalence of kratom use are scarce, but available evidence indicates that its prevalence is on the rise in the United States [15], Europe [16], and developed eastern countries such as Japan [17]. In the USA, over 1800 total calls related to kratom ingestion were received by US poison centers in the 7-year interval from 2011 through 2017, with nearly two-thirds of these occurring in the last 2 years of the period, signifying the rapid rise in the use of the substance [18]. Moreover, a recent synopsis on kratom estimated the number of users in the USA to be in the range of 3–5 million based on membership numbers obtained from the American Kratom Association [19]. If accurate, this would correspond to approximately 0.9–1.5% of the US population reportedly using kratom. This trend is also reflected in the expanding scientific literature, where the number of case reports describing kratom intoxication continue to accumulate [20–23].
Particularly in the West, kratom is often used as a recreational drug, where it is perceived as a safe, “legal high” [12]. This reputation led to the proposed categorization of kratom as a Schedule I drug by the US Drug Enforcement Administration (DEA) in 2016, but it garnered little interest among policymakers. Thus, a key contributor to the problem is that kratom remains unrecognized as a controlled substance by the DEA and is therefore not subject to regulation by the US Controlled Substances Act [24]. Although it is currently listed on the DEA’s Drugs of Concern registry, this is mostly a symbolic measure and does little to prevent its sale. However, as of 2019, six states legislatures (Alabama, Arkansas, Indiana, Wisconsin, Rhode Island, and Vermont) have successfully passed statutes criminalizing kratom possession [25]. In the rest of the USA, it remains legal and is easily obtained in stores or through numerous online retailers. Its sale is permitted throughout Europe as well, with the exception of Poland, Ireland, and Romania, as well as most of the Nordic and Baltic states [26].
To be sure, its unscheduled status and widespread availability have contributed to the expansion of kratom within Western markets [27]. However, in the USA, the more fundamental issue underlying the growing demand for kratom is the current opioid abuse epidemic [28]. As prescribers are pressured to cut back on supplying opioid medications, patients with opioid dependence often resort to alternatives like kratom to support their habit as traditional opioids become scarce [29]. Kratom is also sought out by those who wish to self-medicate for health conditions such as chronic pain or opioid withdrawal/dependence, and it has been heralded as a legal, inexpensive alternative to opioid replacement regimens [30]. The efficacy of kratom for such purposes remains highly questionable, and more research is needed to establish a conclusive answer.
Pharmacology of Kratom and Prospects in Therapeutics and Research
Kratom does not denote a single, specific compound, but rather a cocktail of the psychoactive alkaloids occurring naturally in the plant. More than 40 of these compounds have been identified to date, although only four are known to be pharmacologically active: mitragynine, 7-hydroxymitragynine (7-OH- mitragynine), speciociliatine, and corynantheidine [31]. The most prevalent is mitragynine, which accounts for approximately 2% of kratom preparations by mass, but up to 66% of the total alkaloid content [32]. Its highly active oxidized metabolite, 7-OH-mitragynine, is present in far lower quantities, generally under 0.02% [33]. Other indole alkaloids present in significant concentrations include speciogynine, paynantheine, and mitraphylline [34]. Like the remaining trace alkaloids, these compounds are not known to be pharmacologically active; however, it is possible they may contribute synergistically to the overall effect of kratom in an unknown manner. Given the diversity of alkaloids present in kratom extracts and the unique potential pharmacodynamic properties of each, the net physiological effect of the substance is complex, intermixing stimulant and opiate-like properties in a dose-dependent manner (primarily stimulant-like at low amounts, with opioid effects predominating at higher doses) [35, 36].
Both mitragynine and 7-OH-mitragynine target opioid receptors, albeit with significant differences in binding affinity [37]. In fact, while the affinity of mitragynine for opioid receptors is less than that of morphine, 7-OH-mitragynine is far more potent than either, approximately 46 times that of mitragynine and 13 times that of morphine [38, 39]. Despite considerable investigation, the precise manner in which kratom alkaloids act at each of the receptors remains disputed. For example, Takayama and colleagues have produced a sizeable body of work on the subject, indicating that both mitragynine and 7-OH-mitragynine behave as agonists, with mitragynine acting primarily on µ- and δ-receptors and 7-OH-mitragynine more selective for µ- and κ-receptors [39–41]. However, competing evidence suggests a different model; rather than acting as simple agonists, mitragynine and 7-OH-mitragynine appear to demonstrate variable effects depending on the receptor. Specifically, the data show that both mitragynine and 7-OH-mitragynine are mixed opioid receptor agonists/antagonists, behaving as partial agonists at µ-receptors and competitive antagonists at δ-receptors, with negligible effects on κ-receptors [42].
Importantly, the indole alkaloids in kratom are structurally and pharmacodynamically distinct from their opioid counterparts, producing partially overlapping but nonidentical effects. Accordingly, these compounds have been called atypical opioids to distinguish them from morphine, semisynthetic opioids, and endogenous ligands [43]. Like the opioids, binding of the indole alkaloids to opioid receptors initiates G-protein-coupled receptor (GPCR) signaling; however, unlike traditional opioids, the activation of GPCRs by indole alkaloids does not initiate the β-arrestin pathway [44]. This phenomenon, known as biased agonism or ligand-directed signaling, enables a single receptor to mediate multiple different intracellular effects by selectively disengaging the various signaling cascades coupled to the receptor [45]. Interestingly, β-arrestin recruitment is responsible for most of the symptomology associated with opioid use (e.g., respiratory depression, sedation, constipation) [46, 47]. Thus, the selective inactivation of β-arrestin represents a desirable feature for an opioid, and suggests that mitragynine might be a useful template for designing novel opioids with more tolerable side effect profiles.
In addition to its opioid-like analgesic effects, mitragynine appears to block pain signaling through other mechanisms as well, suggesting a multimodal role in regulating pain perception. For instance, mitragynine shares considerable structural homology with yohimbine, another indole alkaloid, which has well-known adrenergic properties [37]. Like yohimbine, experimental evidence indicates that mitragynine activates α-2 adrenergic postsynaptic receptors [48]. This is significant for mitragynine’s analgesic effects, as α-2 receptors are present in modulatory “descending” pain pathways [49]. The importance of these pathways has only recently become apparent, and represent a major advancement in the complex neurobiological understanding of pain [50]. A third anti-nociceptive mechanism has been proposed in light of evidence that mitragynine impairs neuronal pain transmission via blockade of Ca2+ channels [51]. Additionally, indirect analgesic properties have been attributed to mitragynine’s putative anti-inflammatory effects, secondary to the inhibition of COX-2 and prostaglandin E2 mRNA expression [52, 53]. In addition to these anti-nociceptive functions, mitragynine bears some affinity for receptors in the central nervous system, including the 5-HT2C and 5-HT7 serotonin receptors, D2 dopamine receptors, and A2A adenosine receptors, but the physiological significance of these interactions is unclear [41].
The metabolism of kratom alkaloids is primarily hepatic, with several cytochrome P450 (CYP) isoforms involved, including CYP3A4, with lesser contributions from CYP2D6 and CYP2C9 [54]. It demonstrates linear pharmacokinetics and has a biphasic elimination pattern from the plasma when ingested orally, suggesting a two-compartment model of distribution [55]. The half-life of mitragynine has been reported to be as short as 3 hours, although some studies suggest it may be much longer [56, 57]. A major development in the understanding of kratom pharmacology has been the recognition that mitragynine is converted into 7-OH-mitragynine by hepatic metabolism in vivo [58–60]. Consequently, it has been postulated that 7-OH-mitragynine actually represents the active metabolite of mitragynine, accounting for most or all of the effects traditionally attributed to the mitragynine precursor. This hypothesis was first described by a trio of 2019 publications conducted by three separate groups [58–60]. These studies provided evidence that the activation of mitragynine occurs by CYP34A-mediated dehydrogenation—a process analogous to the activation of opiates such as codeine, which is converted into is active metabolite by CYP2D6. Although 7-OH-mitragynine is present in kratom extracts, it occurs at trace concentrations, leaving the authors to conclude that any ingested 7-OH-mitragynine is inconsequential relative to the endogenous generation of 7-OH-mitragynine derived from mitragynine. As current work is limited to animal models, future studies will need to confirm the relevance of this discovery in human physiology.
Effects of Kratom Alkaloids In Preclinical Studies
Concern for the potential adverse effects associated with kratom has led to numerous preclinical investigations on the subject, such as the risk for dependence and addiction posed by mitragynine and related alkaloids. For instance, both mice and rat models have demonstrated addiction potential and cognitive impairment particularly in the setting of chronic mitragynine ingestion [61–63]. Studies also have found that the development of addiction and toxicity is specifically dependent on 7-OH-mitragynine, with mitragynine posing a minor risk [61, 64]. Moreover, chronic use has been associated with enhanced punishment tolerance and reward-seeking behavior [65]. Despite these adverse properties, animal model studies have also identified possible benefits; for example, mitragynine appears to slow the development of opioid tolerance when co-administered with morphine in mice, an observation which raises interesting possibilities for clinical applications [66].
Kratom has also been implicated as a cause of organ dysfunction and toxicity [67]. Animal studies have indicated a risk for drug–drug interactions, namely through modulating hepatic P450 activity and drug metabolism [68, 69]. Mitragynine also appears to inhibit hepatic demethylases and transferases, as well as glucuronidation by UDP-glucuronosyltransferases (UGT) such as UGT2B7 and UGT1A1 [70–73]. This bears important implications for a possible interaction when kratom is co-administered with other drugs known to be UGT substrates (e.g., buprenorphine and ketamine, metabolized by UGT2B7) [73]. Such findings have been used as a potential explanation for cases of toxicity following co-ingestion of kratom with other medications, including a reported fatality secondary to toxicity from supratherapeutic levels of a prescribed antipsychotic concurrent with kratom ingestion [74]. The authors attribute this outcome to a drastic reduction in clearance of quetiapine (a CYP3A4 substrate) secondary to the acute suppression of hepatic metabolism by kratom.
Clearly, the basic science literature raises legitimate concerns regarding the potential for drug toxicity and behavioral risks following kratom ingestion. However, a major limitation of the preclinical literature is that many of the experiments were conducted using either chemically synthesized mitragynine or 7-OH-mitragynine rather than actual kratom (although a few studies utilized kratom leaf methanolic extracts) [75–77]. Consequently, such evidence likely represents an oversimplified and incomplete portrayal of the possible effects attributable to actual kratom consumption. This fundamental distinction must be considered prior to drawing any conclusions about patient safety from preclinical investigations.
Potential for Addiction and Toxicity
As alluded to earlier, the historical record concerning kratom’s potential for dependence and addiction in humans raises strong concerns about its safety [41, 62, 78]. However, in many cases the primary motivation among regular users may simply be as a means to prevent exhaustion, and improve energy or mood. In such cases, routine use may not constitute dependence or addiction per se, but rather merely the desire to improve productivity [9]. This is in alignment with “drug instrumentation” theories, in which a substance is utilized in a purposeful, goal-directed manner [79, 80]. Such theories may account for the low incidence of kratom use disorder and other side effects among traditional users in Southeast Asia [81–85]. Nevertheless, the successful instrumentation of kratom does not preclude the potential for prolonged drug use, which under certain circumstances can degenerate into outright addiction [78]. It has also been proposed that a significant amount of kratom use occurs as a substitute for more harmful substances (namely narcotics) in patients with existing substance abuse, in which case kratom use represents a sort of harm reduction rather than drug abuse [79, 86]. Yet, while there is convincing evidence that kratom has significantly less potential for dependence and overdose than traditional opioids, the use of kratom in place of established medical opioid replacement regimens has little basis in evidence [30, 87, 88].
Aside from its potential for abuse, kratom poses numerous others risks to patients, largely a consequence of its status as an unregulated supplement. Without regulatory oversight, there is little to ensure the authenticity, purity, quality, potency, and safety of commercially available kratom preparations [89]. Consequently, it is difficult to know for certain what is actually present within commercially available kratom preparations, and the concentration of mitragynine contained can vary considerably [90]. For instance, it has been reported that kratom products may be altered by artificially increasing levels of 7-OH-mitragynine to enhance potency [91]. In addition, multiple instances of deliberate adulteration of kratom have been documented, for instance, by adding synthetic substances such as phenylethylamine (PEA) or O-desmethyltramadol, both of which have resulted in patient deaths [92, 93]. Other risks include product contamination (intentional or otherwise). For example, laboratory and epidemiological evidence identified kratom as the source of a multi-state salmonella outbreak in 2018 [94, 95]. There have also been cases describing the sale of kratom products later found to contain harmful heavy metal contaminants [96]. As there is considerable disparity between reported kratom toxicities in the West and in Southeast Asia (where it is comparatively uncommon), it has been suggested that misinformation regarding the content and potency of kratom may be largely responsible for the apparent danger attributed to kratom use [36].
Clinical Presentations of Kratom Abuse
Seeking to gauge the spectrum of possible symptoms associated with kratom toxicity, a 2019 retrospective review of cases reported to the National Poison Data System and New York City Office of the Chief Medical Examiner identified a wide variety of presenting symptoms, with agitation being the most common at 18.6%, followed by tachycardia at 16.9%, drowsiness at 13.6%, and confusion at 8.1% [97]. Serious neurological sequelae included seizures in 6.1% of cases, and hallucinations in 4.8%, with 2.3% progressing to coma. Toxicity occurred in a dose-dependent manner, particularly when doses of kratom powder exceeded 8 g. The study also determined kratom to be a contributing factor in at least four deaths. Consequently, the authors concluded that kratom supplements pose a public health risk and should not be presumed safe despite being legal for purchase.
Case studies reveal that a wide range of organ systems are susceptible to kratom-mediated injury (Table 1). For example, instances of kidney injury [67], cardiotoxicity and arrhythmia [98, 99], thyroid injury and hypothyroidism [100] lung injury/acute respiratory distress syndrome (ARDS) [101, 102], neonatal abstinence syndrome, [103–107] and hepatic injury [23, 108–116] have all been linked to kratom. Hepatic injury is an especially common presentation, and often presents with a cholestatic hepatitis pattern similar to other drug-related injuries: transaminitis (usually with levels above 100 units/L) along with an elevated alkaline phosphatase (> 200 units/L) and total bilirubin (> 1.2 mg/dL). A variety of neurological complications due to kratom toxicity have also been described, including acute brain injury and coma [21], along with the risk of seizures in both the acute and chronic setting [117, 118]. Long-term cognitive impairment may develop after long-term chronic users [81].
Table 1
Spectrum of organ system involvement and corresponding injuries associated with kratom use as identified in the case study literature
| Organ system | Presentation signs and conditions | References |
|---|---|---|
| Hepatic | Acute liver failure, hepatitis, transaminitis, intrahepatic cholestasis, hepatomegaly | [23, 108–116, 131] |
| Endocrine | Hypothyroidism, hypogonadism | [26, 100] |
| Renal | Acute kidney injury | [67] |
| Cardiac | Cardiotoxicity, arrhythmia | [98, 99] |
| Pulmonary | Acute lung injury, ARDS | [101, 102] |
| Obstetric | Neonatal abstinence syndrome | [103–107] |
| Neurological | Acute brain injury, seizure, coma, cognitive impairment | [21, 81, 117, 118] |
ARDS acute respiratory distress syndrome
In certain severe cases, kratom toxicities have resulted in death. In fact, the incidence of kratom-related mortality appears to be rising, according to reporting by the Centers for Disease Control and Prevention (CDC), which linked kratom to 152 deaths between 2016 and 2017 [96]. Importantly, the existence of polysubstance abuse is a key risk factor predisposing patients to toxicity and death and has been estimated to occur in 87% of cases [119]. This has led to the belief that death resulting solely from ingestion of kratom is exceedingly rare, even impossible. However, in a 2019 article assessing kratom-related mortality in the state of Colorado, the authors reported that at least 4 of the 15 total deaths between 1999 and 2017 were attributable exclusively to mitragynine toxicity, a result which the authors confirmed using an extensive toxicological and biochemical workup [120]. Nevertheless, it remains probable that most kratom-related deaths are the result of kratom toxicity superimposed upon the effects of some other noxiousness factor, such as adulterants or contaminants within the kratom product itself, or in conjunction with the ingestion of another illicit substance.
Read more about Kratom Pharmacology
Peaple also love: MIT 45 Kratom and Chief Kratom Extract Liquid Shots. For Kratom Powder Lovers, Remarkable Herbs Kratom
0