An abbreviation consisting of the initials of Good Manufacturing Practices forms the GMP term. While Good Manufacturing Practices has begun with cosmetics industry for the first time, today it covers production in healthy conditions and safe environments and goods made in many fields related to people's health, such as medication, medical devices, and equipment, food, and cosmetics.
Not only products and staff, but also every aspect of industrial companies is controlled by this system. Under this context, the manufacturing facilities, the machinery, and equipment utilized, the environmental conditions, the production procedures, the specifications of the raw materials used, the skills and experiences of the workers are all factors that determine the product's efficiency and quality.
In terms of public health, producers operating in industries such as pharmaceuticals, food, and cosmetics are largely responsible for eliminating or at least mitigating the possibility of contamination of goods from various sources. The required actions are required in this regard, with the legal regulations made in each country. The important thing is that these items are manufactured under reliable conditions and specified specifications.
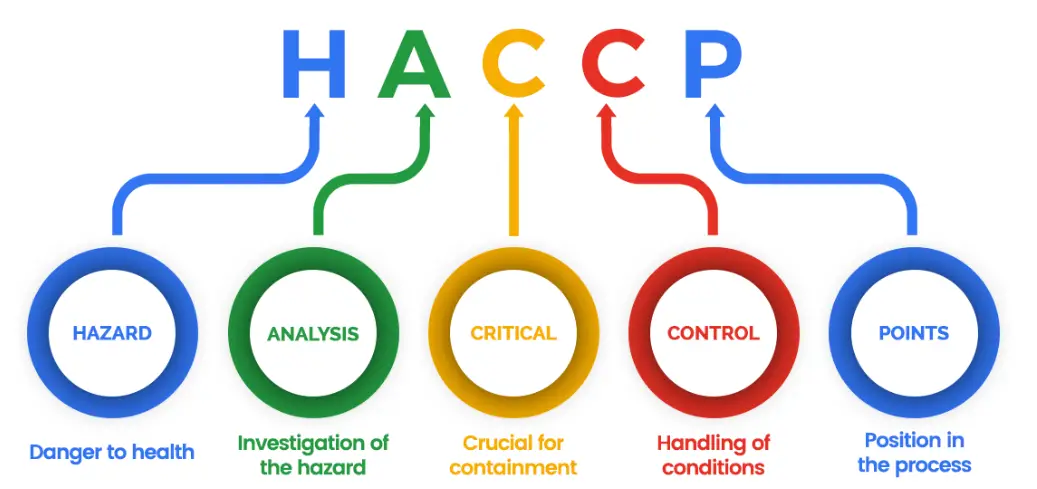
Today, the sectors in which the GMP Certification is used have greatly increased. ISO 9001 Quality Management System, ISO 14001 Environmental Management System, HACCP Hazard Analysis and Critical Control Points System, ISO 22000 Food Management System, OHSAS 18001 Occupational Health and Safety Management System, SA 8000 Social Responsibility System, ISO 16949 Quality Management System for the Automotive Industry and ISO 13485 and 13488 Management System for Medical Devices included within the scope of GMP. All these programs provide implementations at the international level for the development and protection of health, environment, food hygiene standards.
What are the requirements of GMP?
As mentioned above, the GMP Good Manufacturing Practices Framework is concerned with both the industrial and quality management aspects of healthy products. It is important to evaluate and clearly define all processes in the stages of production. The goods must comply in all circumstances stated within the specifications.
The healthy application of the scheme relies on the provision of each risk.
– Staff members must, for example, be educated and qualified, the operating environment should be acceptable for manufacturing, relevant and appropriate materials should be used in manufacturing.
– The suitable industrial machinery ought to be accessible in the organization. Application guidelines, and workflow concepts should be planned, sufficient storage facilities should be available for the goods produced and transported.
– It is necessary to authorize processes considered to be essential during development activities and to make improvements to those processes.
– For the records of manufacturing activities indicating that the works are conducted in compliance with the regulations and also workflow charts, a good and reliable storage method should be applied.
– If there is any anomaly observed, it must be documented.
– The documents held should be open to the access of necessary authorities and persons.
– Different risks that can impact the quality of goods should be eliminated from the delivery methods and processes.
– Where goods have to be withdrawn from the market in unforeseen circumstances, an order to facilitate this process should be enforced in the organization as a rapid response.
To receive, review, and respond to potential customer complaints, a system should be set up. Companies gain major benefits from this when all these criteria are met. The valuation and prestige of the company would increase significantly when they have GMP certification. At the very same time, the organization will become equipped for the required official inspections since they have already realized activities necessary to the procedures and legal regulations.
If you are looking for GMP certification in Malaysia, then Mandreel would be the best choice.