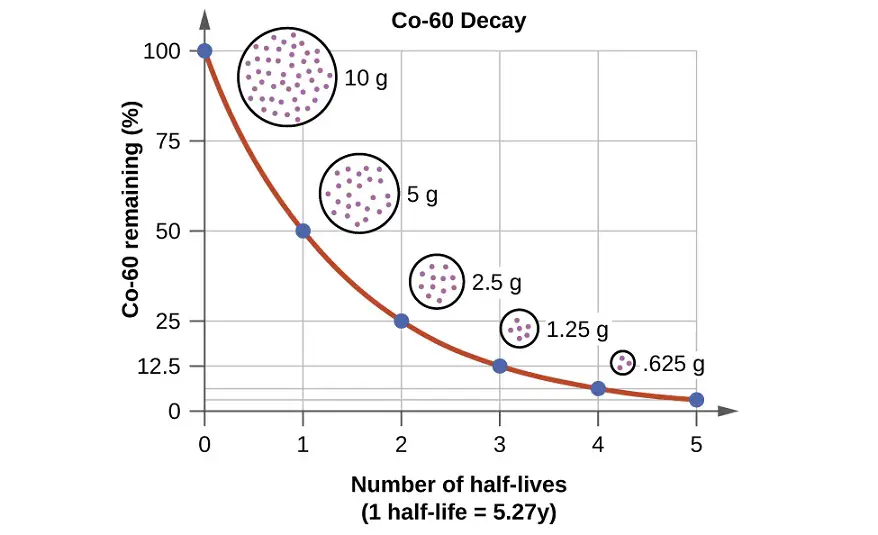
During a natural radioactive decay, not all the atoms of an element are suddenly changed to atoms of another element. The decay process takes time and there is value in being able to express the rate at which a process occurs. It is described by a phenomenon called Half-life is the time required to reduce the number of atoms half to its original value. The term is also used for any type of exponential or non-exponential decay.
Moreover, Half-life is constant over the period of an exponentially decaying value, and it is a typical unit to represent the exponential decay equation.
Radioactive decay and its types
Radioactive decay is also known as nuclear decay, radioactivity, or radioactive disintegration, it is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered radioactive. Three of the most common types of decay are alpha decay, beta decay, and gamma decay, all of which involve emitting one or more particles or photons. Radioactive decay is the random process at the level of single atoms. According to the quantum theory, it is impossible to forecast when a specific atom will decay, irrespective of how long the atom has existed. But, for a significant number of same atoms, the overall decay rate can be stated as the decay constant or half-life. The range of half-life is different for different elements, it ranges from an instant to hundreds of years.
The Half-life calculator to find half-life in decays
The half-life calculator helps you to calculate the half-life more effectively. It uses the formula of half-life to calculate the number of remaining atoms, decay time and the total number of atoms. Half-life formula and the equation is described below.
Half life formula
Any exponential decay can be described by any of the following three corresponding formulas:
N (t) = N0 (1/2)t/t1⁄2
N (t) = N0et/τ
N (t) = N0 e- λ t
note that
-
N0 = initial quantity of the substance that will decay (it may be in grams, moles, number of atoms, etc.),
-
N(t) = the quantity that still remains after a time t,
-
t1⁄2 = the half-life of the decaying element,
-
τ = a positive number which is called the mean lifetime of the decaying element,
-
λ = a positive number which is called the decay constant of the decaying element.
The three parameters t1⁄2, τ, and λ are related in the following way:
t1/2 = ln(2) = ln(2)
where ln(2) is the natural logarithm of 20.693
How to use half-life calculator?
It is very easy to calculate the half-life with a calculator. Our calculator uses very basic techniques to calculate the half-life. All you need is to follow the following steps in order to calculate your values easily.
-
Enter the initial amount of element/substance in the first field.
-
Enter the final amount of element/substance in the second field.
-
Enter the time taken by the element/substance to decay.
-
Now please click on the “CALCULATE” button.
You get your answer immediately after clicking the calculate button. You can use the above-described formals to calculate the values manually to confirm your answer.